Tag Archives: Biol 1362
AMINO ACIDS AND PROTEINS 2!!!
REFLECTION #6
Boy class today was really packed! I reached late, but I made it 🙂 So continuing amino acids and proteins as promised. Today’s class had a lot of info packed in. Had so much to take in feels like…

But don’t worry you’ll like this topic as long as you understand whats going on. My lecturer taught this topic really good, the only suggestion I could give is to include more visual aids, that always helps me. Oh and keep explaining things on a simple level like you do Sir, makes it whayyy easier to understand 🙂
WHAT ARE PROTEINS??

A Protein is a biological macromolecule of molecular weight 500g/mol or greater, consisting of one or more polypeptide chains.
Functional classes of proteins
- Receptors- sense stimuli
- Channels- control cell content
- Transport- eg. hemoglobin in blood
- Storage- eg. ferritin in liver
- Enzyme- catalyze biological reactions
- Structural- collagen in skin
- Immune response- anti-bodies
There are many type of proteins:
- Globular proteins
- Fibrous proteins
- Membrane proteins
LEVELS OF PROTEIN STRUCTURE
PRIMARY STRUCTURE
The primary level of protein structure is not just the number and identity of the component amino acids in the protein, but the order or sequence in which the specific amino acids are combined (by condensation, formingpeptide bonds) in the polypeptide chain.
SECONDARY STRUCTURE
The polypeptide chain can fold back on itself in a number of ways. The polypeptide chain is coiled into a helical structure called the alpha helix, in which interactions take place between groups 3 – 4 amino acid residues apart. Some amino acids put a kink or bend in these regions of helical structure, allowing the chain to bend back on itself and form a more globular molecular structure. The beta pleated sheet is another regular secondary structure.
TERTIARY STRUCTURE
The 3-dimensional structure of a protein’s polypeptide chain or chains may be locked in place by other stronger bonds. These bonds are formed between components of the -R groups of the amino acid residues. The types of bonds may include: Ionic bonds, hydrogen bonds. disulphide bridges, hydrophobic forces and covalent links to other groups.
QUATERNARY STRUCTURE
A protein with a quaternary structure consists of more than one practically identical sub-unit, not joined by strong bonds like those above.
A well known example is haemoglobin, which consists of 2 alpha and 2 beta chains, consisting of 141 and 146 amino acid residues respectively.
An awesome Amino acids and Proteins video
Another good vid.
REFERENCES
http://greatergood.berkeley.edu/ei_quiz/
http://www.bizarrebytes.com/bizarre-headlines-in-the-news/
http://www.biotopics.co.uk/JmolApplet/proteinjstructure.html
AMINO ACIDS AND PROTEINS!!
REFLECTION #5
Amino acids and Proteins is a very long complex topic. Lets hope I can make this a little interesting as well as understandable. Class was good, cold but good. My lecturer made this stuff easy. He can really break down complex stuff into ABC. So lets get started….. 🙂
Amino acids is used to make proteins during protein synthesis. Amino acids is relatively small molecules. Glycine being the smallest. What makes amino acids different from each other is the R-group attached to the chiral carbon.

So just how important is amino acids to us???

Well there are 20 essential amino acids which makes proteins in our bodies that is needed from the food we eat, for growth and health. There are essential and non-essential amino acids, however both are needed and just as important. The essential amino acids will be the ones our body can not produce. For example: arginine, histidine, isoleucine and leucine. The non-essential amino acids will be the ones needed for making important proteins in the body, but the body can actually make these. Some example: alanine, asparagine, aspartic acid and cysteine.
NINHYDRIN REACTION
This is a test for amino acids. The ninhydrin and amino acid react to give a purple coloured imino derivative.
PROTEIN TEST: BIURET

Biuret is a light blue solution (containing copper 2+ ions) which changes purple when it reacts with a protein. This is because the copper ions reacts with the peptide bonds to form a complex.
What is a peptide bond??

A peptide bond is a chemical covalent bond formed between the α-amino group of one amino acid and the α-carboxyl group of another.
So your wondering is that all for amino acids and proteins…..

Well nooooooo!!!! there is another blog on this topic coming soon…so make sure to look out for that 🙂
REFERENCES
http://www.proprofs.com/flashcards/story.php?title=biological-molecules-aqa-proteins
http://www.carnicominstitute.org/articles/proteincomplex.html
http://www.encyclopedia.com/topic/biuret_test.aspx
http://aam.govst.edu/projects/jshileny/student_page_communication.htm
http://www.blc.arizona.edu/courses/schaffer/182/peptidebond.HTM
http://www.liberalrev.com/?p=3334
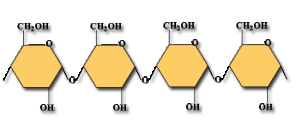
CARBOHYDRATES 2!!!
REFLECTION #3
Well as promised, today I will be continuing the yummy topic of carbohydrates.
DID you know there are many types of carbohydrates?
THE DIFFERENT TYPES OF CARBS:
- Monosaccharides– is a simple sugar with multiple OH groups
- Disaccharides– two covalently linked monosaccharides
- Oligsaccharides– a few monosaccharides covalently linked
- Polysaccharides- polymers consisting of chains of monosaccharide units
MONOSACCHARIDES
Monosaccharides can have different functional groups at the end . For example, aldoses have an aldehyde group at the end, whereas ketoses have a keto group at the end. This makes all the difference.
STRUCTURE OF D-GLUCOSE

STRUCTURE OF D-FRUCTOSE

Do you know what chiral objects are??
Well chiral carbons or asymmetric carbons have 4 different groups attached.
 Glucose has many stereoisomers. For example, there is D-Glucose and L-Glucose. The D & L designations are based on the configuration about the single asymmetric C. For sugars with more than one chiral carbons, the D & L refers to the asymmetric carbon furthest form the aldehyde or keto group.
Glucose has many stereoisomers. For example, there is D-Glucose and L-Glucose. The D & L designations are based on the configuration about the single asymmetric C. For sugars with more than one chiral carbons, the D & L refers to the asymmetric carbon furthest form the aldehyde or keto group.
HEMIACETAL & HEMIKETAL
Hemiacetal- is formed when an aldehyde reacts with an alcohol
Hemiketal- is formed when a ketone reacts with an alcohol
Sugar devivatives!
When a sugar lacks an adehyde or keto group it is call a sugar alcohol.
However a sugar acid is formed when the aldehyde on C1, or the OH at C6 is oxidized to carboxylic acid.
When an ammino group substitutes for a hydroxyl group an Amino sugar is formed.

YES I KNOW IT IS A LOT TO LEARN!!! BUT YOU WILL MAKE IT!!
Well honestly this topic was not a lot of fun. But its up to me and you to make to it fun. The lecture could be improved by more visual aids.
CARBS VID
REFERENCES
http://commons.wikimedia.org/wiki/File:D-glucose.png
http://www.starch.dk/isi/starch/fructose.asp
http://www.rpi.edu/dept/bcbp/molbiochem/MBWeb/mb1/part2/sugar.htm
http://guweb2.gonzaga.edu/faculty/cronk/CHEM198pub/L07-index.cfm
CARBOHYDRATES!!!!
REFLECTION #2
We all love eating carbohydrates aka the comfort food!!
Carbs make us feel all nice in the tummy, it is a great source of energy.
In class today I have learnt the Biosignificance of Carbohydrates. It turns out that it is much more than just an energy source. 
Yep thats right…and we are gona learn all about it in today’s blog.
Also, I’ve learnt about carbohydrate metabolism, that is, how the carbs are broken down by the body to give you that energy you need. 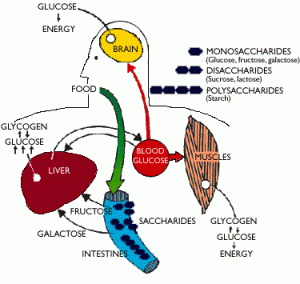
Well lets get strated….
But first we must learn what is a carbohydrate:
Carbohydrates are called carbohydrates because they are essentially hydrates of carbon, that is, they are composed of carbon and water. it has a composition of (CH2O)n.
THE BIOSIGNIFICANCE OF CARBOHYDRATES:
- Energy source
- Storage
- Structure
- Precursor molecule
So lets go into each one of these a little….
ENERGY SOURCE-
When we eat carbs, during metabolism, it is broken down to carbon dioxide and water. I am sure you are familiar with the equation for respiration: C6H12O6 + 6O2 >>>> 6CO2 + 6H2O + energy Monosaccharides and disaccharides would break down quickly which makes it a quick energy source. However, starches take longer to break down, but the end product is still the same. There is one type of carbohydrate humans can’t metabolize, and that is cellulose. We can not break it down because we don’t have the appropriate enzyme to breakdown the β(1-4) linkage in cellulose.
STORAGE-
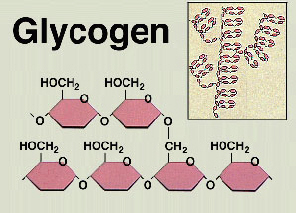 When you eat too much and got a high glucose concentration,
When you eat too much and got a high glucose concentration,
the body needs a way to store this glucose for a rainy day.
So the pancreas comes to the rescue, producing insulin
which will convert the glucose into glycogen.
This glycogen is then stored it in the liver and muscles. 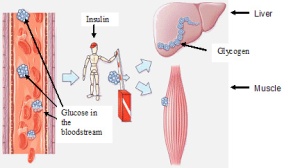 However. when the glucose concentration is low again, the hormone glucagon will convert glycogen back into glucose.
However. when the glucose concentration is low again, the hormone glucagon will convert glycogen back into glucose.
Plants in the other hand, store their glucose as another carbohydrate complex called Starch.
STRUCTURE-
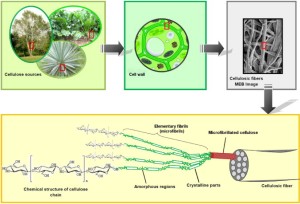 Plants use cellulose as a component in
Plants use cellulose as a component in
their cell walls, because it is a polymer of
β-D-hlucose which forms strong fiber and
is a very good building material.
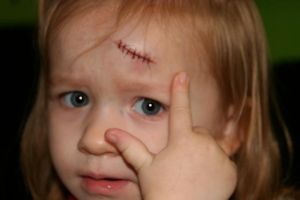
Chitin is also a structural polysaccharide and is found in the exoskeletons of some insects. 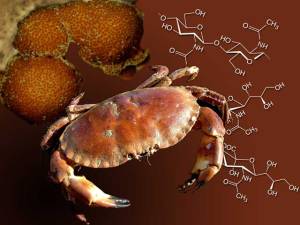
Chitin is often used in medicine as well, for its structure as it is both strong and flexible. Did you know that when you get a cut and you go to the doctor and he stitches you up, those Stitches is made of chitin.
PRECURSOR MOLECULE-
You may be wondering… precursor molecule??? WHAT IS THAT!!!
Well a precursor molecule is a molecule that is the chosen starting point for the preparation of another molecule.
Carbohydrates are precursors for the synthesis of certain biomolecules. For example; ribose form part of the skeleton of DNA and RNA. It also serves as raw material for the synthesis of small organic molecules, for example; ammino acids and fatty acids.
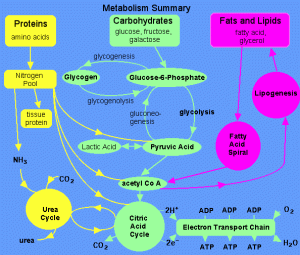
CARBOHYDRATE METABOLISM
Carbohydrate metabolism:
- Glycolysis
- Pentose phosphate pathway
- Glycogen synthesis and catabolism
- Gluconeogenesis
The sole energy source for the brain and nervous system is GLYCOSE which is a type of carbohydrate. Therefore it is important that you include it in your diet to ensure the brain get its source of glucose.
Carbohydrates lesson was very interesting because my lecturer likes to make funny comments on the topic to keep us awake, as well as engage us in class. My only suggestion for the teaching of carbohydrates is that more visual aids could be used to help students follow along in class. Especially the metabolism of carbohydrates could get confusing if you don’t understand whats really happening.
CARBS VID
Well in my future blog post, I will be continuing the discussion of Carbohydrates. So you can look out for that…coming soon… 🙂
REFERENCES:
http://lexicon.dansukker.com/print_expl.asp?id=75
http://kidshealth.org/kid/stay_healthy/food/carb.html
http://www.extension.iastate.edu/families/content/carbohydrate
http://web.visionlearning.com/custom/chemistry/custom/CHE2.1-pg-starch.shtml
http://www.sciencedirect.com/science/article/pii/S014486171200447X
http://www.robaid.com/bionics/turning-chitin-into-pharmaceuticals-with-fungi.htm
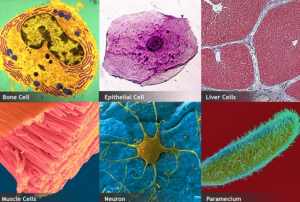
CELLS!!!
Reflection 1
Cells is something everyone has been learning about since high school, or even earlier. Every time I learn about cells, it just keeps going deeper into the processes carried out by the cell and its organelles. In class today I’ve learnt how cell size is determined, the difference between prokaryotes and eukaryotes, and the functions of each organelle in both plant and animal cell.
First we must know what is a cell.
A Cell is the smallest unit that is capable of performing life functions; growth, metabolism, stimulus responses and replication.
As cell size increases, the ratio of surface area to volume decreases. Therefore, the rate of diffusion slows down thus a cell can not get rid of waste fast enough or get nutrients fast enough, so it will either be poisoned by its waste or starve.
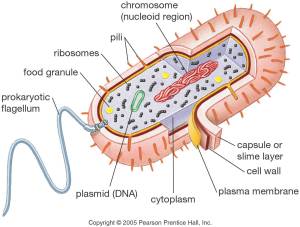
PROKARYOTIC & EUKARYOTIC CELLS
Prokaryotes are single celled organisms that lack nuclei and other organelles. It belongs to the Kingdom Monera, and represented by bacteria and cyanobacteria.
Some of the characteristic features of Prokaryotes are listed below:
- Size: is relatively small about 0.5 micrometers.
- Genetic material: DNA is circular and found in the cytoplasm.
- Organelles: few present (non-membrane bound).
- Cell walls: rigid formed from glycoproeins (mainly murein0.
- Ribosomes: 70s
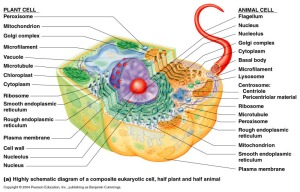
EUKARYOTIC CELLS have a true nucleus and other organelles, such as mitochondria.
Some of the characteristic features of Eukaryotic cell is listed below:
- Size: up to 40 micrometers.
- Genetic material: DNA is formed of linear chromosomes (in nucleus).
- Organelles: Many organelles: double membranes for example in the nucleus, mitochondria and cytoplasm, single membranes for example in the G.A., E.R. and lysosomes.
- Cell wall: Fungi- rigid, formed from polysaccharide and chitin. Plant- rigid, formed from polysaccharides, for example cellulose. Animal- no cell wall.
- Ribosomes: 80s
What I loved about the teaching of the cells, by my lecturer, is that he used a lot of diagrams while explaining the functions of the organelles. This made it easier to remember as well as understand the lesson being taught. I can not fully understand a topic until I am able to visually see whats happening. I would definitely encourage the use of more diagrams when teaching cells. Normally I find the topic of cells to be boring, but I was actually wide awake in class, and its because the class was so interactive, which is another good teaching technique used by my lecturer to keep his students engaged in class.
REFERENCES:
http://en.wikipedia.org/wiki/Eukaryote
http://en.wikipedia.org/wiki/Prokaryote
MY OFFICIAL INTRODUCTION
Hello world,
My name is Kamine Saroop, I am a current student at the University Of The West Indies, pursuing a degree in Biochemistry. I fine Biochemistry to be such an interesting science. My first week into Biochemistry was exciting, I love the teaching methods of my Biochem lecturer, Mr. Jason Matthew. I am crazy about science, it has always been my addiction.I hope to contribute to the world of science one day, as a biochemist. I love nature and I find the world to be such a beautiful fascinating place, I would consider it to be heaven. I like appreciating the simple things in life, I have never found spending time around nature to be a waste of time.